SMART OS 2.4
New SMART OS™ 2.4
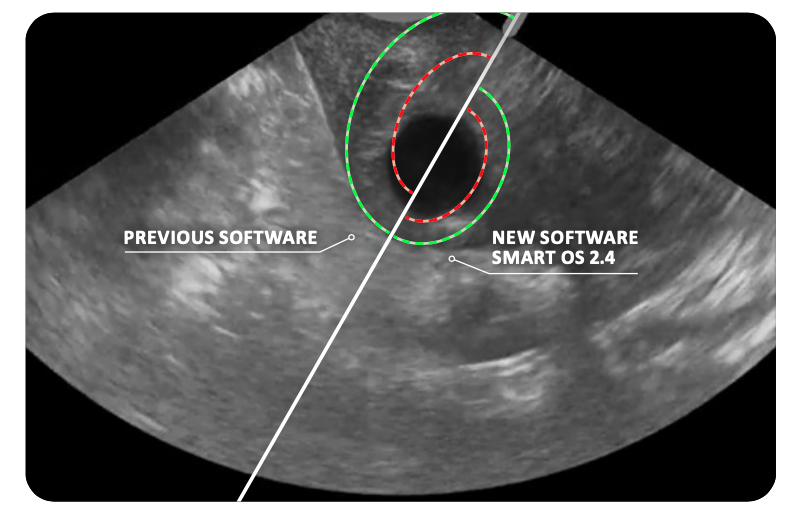
Enhanced SMART Guide with the new SMART OS 2.4 Software
The Sonata System features the SMART guide, a graphical user interface that displays the Ablation Zone directly over the intended treatment area on the ultrasound display.
It's the only system with a Thermal Safety Border that automatically adjusts relative to the Ablation Zone, indicating the distance at which tissue is safe from potential of thermal damage.
SMART GUIDE: Setting Margins of Ablations in Real Time
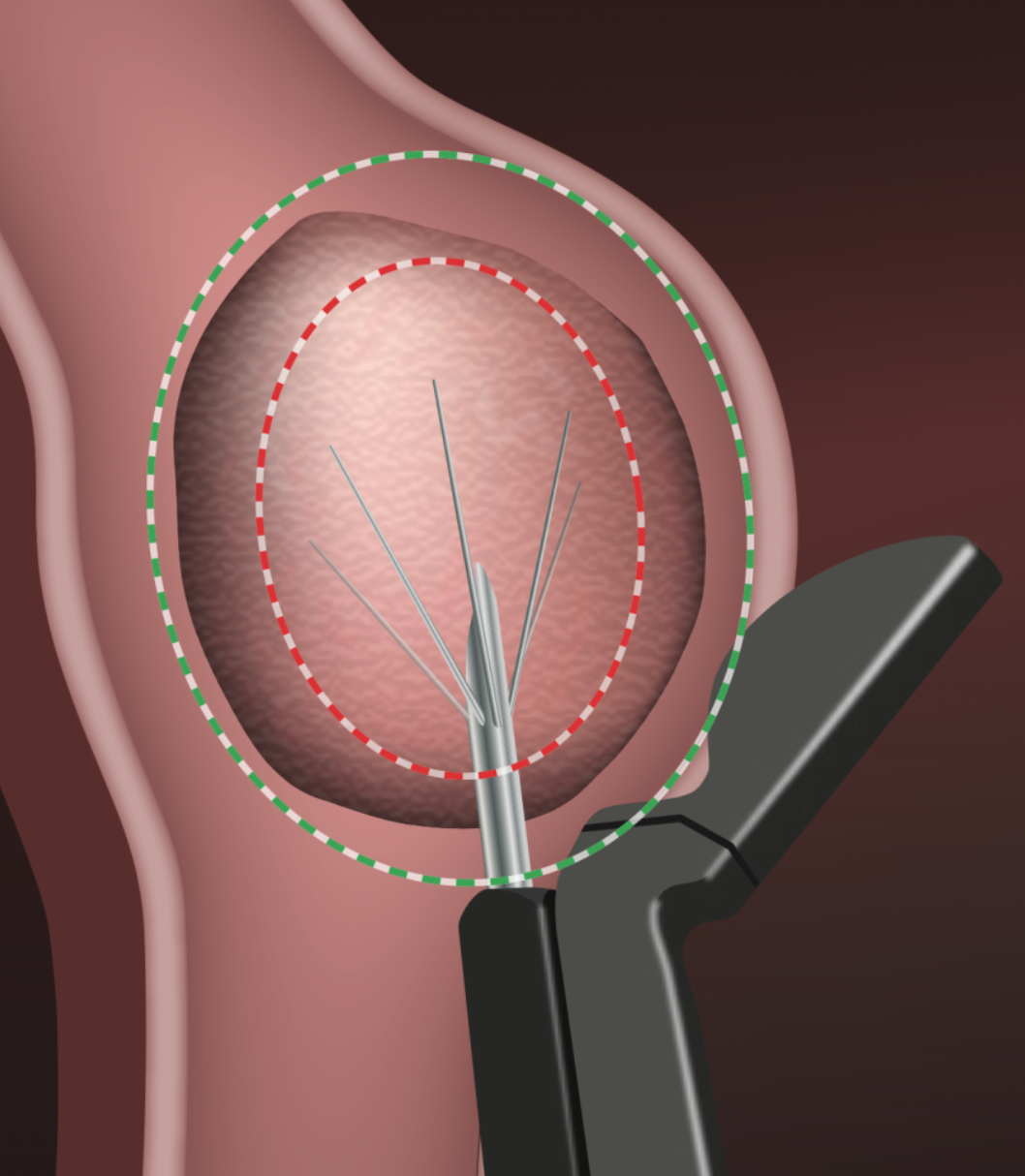
THE PROPRIETARY SMART GUIDE
- Provides a graphical overlay on a live ultrasound image to avoid effects on tissue beyond the Thermal Safety Border.
- Immediately updates the Thermal Safety Border as the physician adjusts the planned Ablation Zone.
- Automatically controls RF delivery to achieve the planned ablation size without the need for calculation or look-ups.
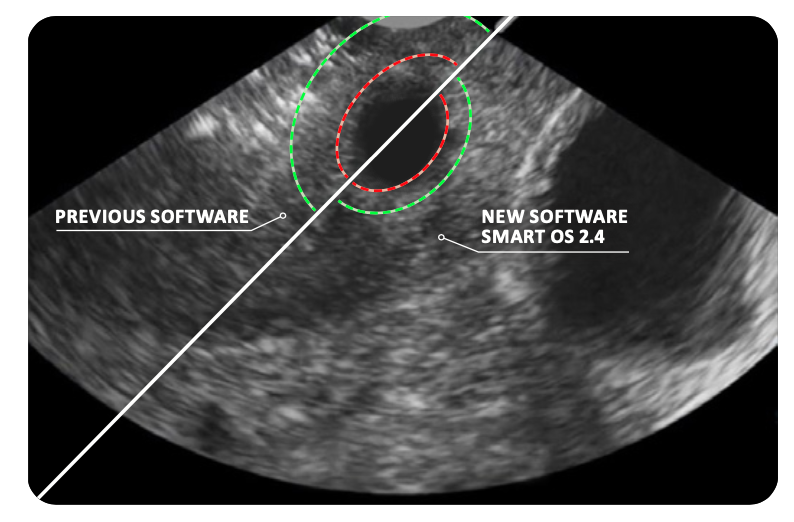
SMART OS 2.4 IMPROVEMENTS OF THE SMART GUIDE:
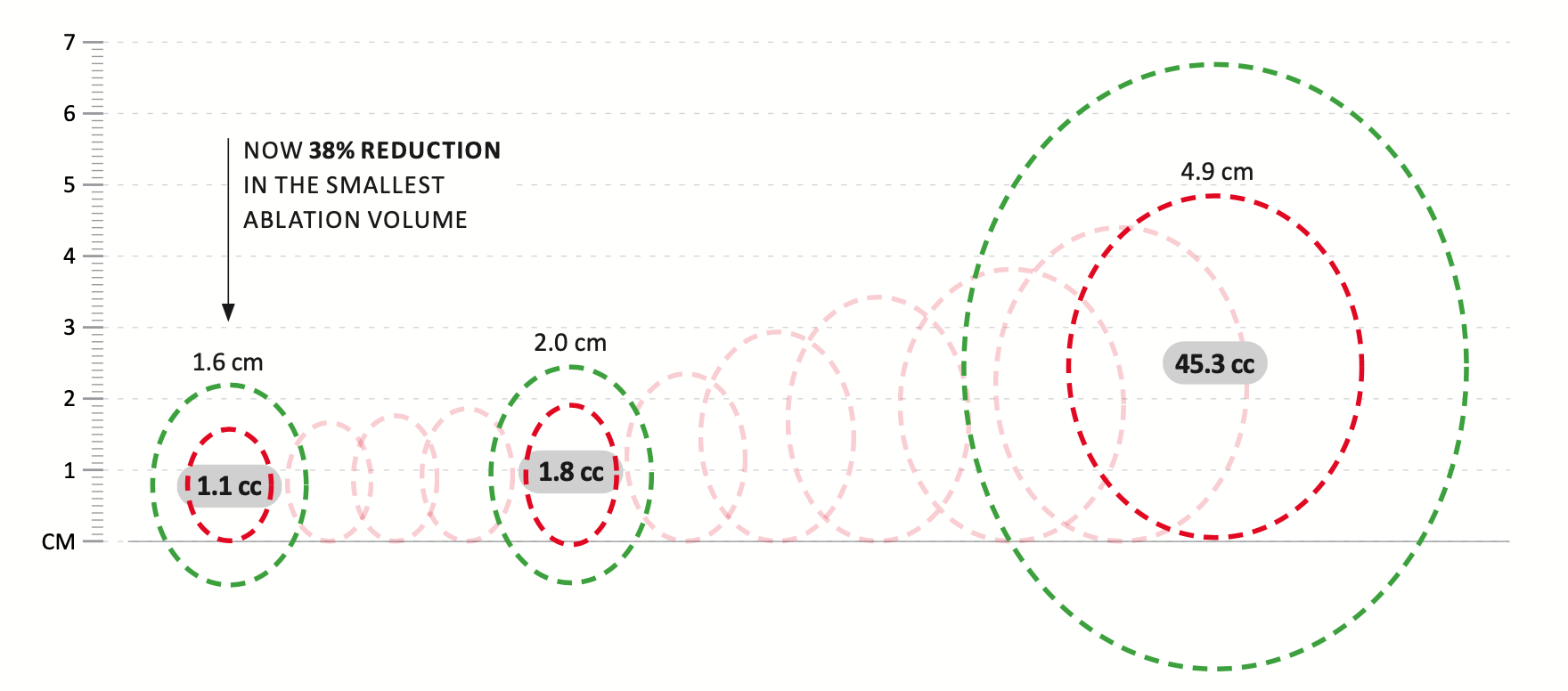
- Reduced smallest Ablation Zone from 2.0 x 1.3 cm to 1.6 x 1.2 cm
- 38% ablation volume reduction
- Optimized margin of Thermal Safety Border for smaller ablation sizes
Precision. Control. Flexibility.
With the new SMART OS 2.4 software for the Sonata System, you are able to treat more fibroids, while preserving normal uterine tissue.

MORE PRECISION FOR INTRAMURAL FIBROIDS
Experience greater flexibility and versatility in making controlled ablations confined to the fibroid, avoiding adjacent uterine tissue if desired.
MORE CONTROL FOR SUBSEROUS FIBROIDS
Treat small (<2-3 cm type 5 (subserous) fibroids more completely, while still containing the Thermal Safety Border within the serosa.
MORE FLEXIBILTY – TREAT A WIDER RANGE OF FIBROID SIZES
Sonata is the only fibroid ablation system that has a Thermal Safety Border, which allows gynecologists to graphically tailor the desired ablation to the targeted fibroid. Sonata provides a 40-fold range from the smallest to the largest ablation volume.
Want to learn more about the Sonata System?
Safety Information | Impressum | Terms of Use | Careers | Contact Us |
Privacy Notice | Cookie Notice | Do not Sell/Share my Personal Information |
Limit the Use of My Sensitive Personal Information
Gynesonics, Inc. | 600 Chesapeake Drive | Redwood City, CA 94063
Copyright 2026 Gynesonics | WS 05195-001 Rev H
