Sonata Fibroid Treatment
Transcervical Fibroid Ablation for Treatment of Symptomatic Uterine Fibroids
Alternative to Hysterectomy & Myomectomy
Does Not Require General Anesthesia
Treats Most Fibroid Types

Incisionless Fibroid Treatment
The Sonata System combines real-time intrauterine ultrasound guidance with targeted radiofrequency ablation in an incisionless procedure to treat symptomatic uterine fibroids.
The Sonata procedure typically takes less than one hour.
On average, women return to normal activity in a couple of days.
How it Works
The Sonata Treatment uses a handpiece with a miniature high-resolution ultrasound tip that allows the doctor to see the fibroids from inside the uterus during the procedure, and then treat each fibroid with a process called an “ablation.”
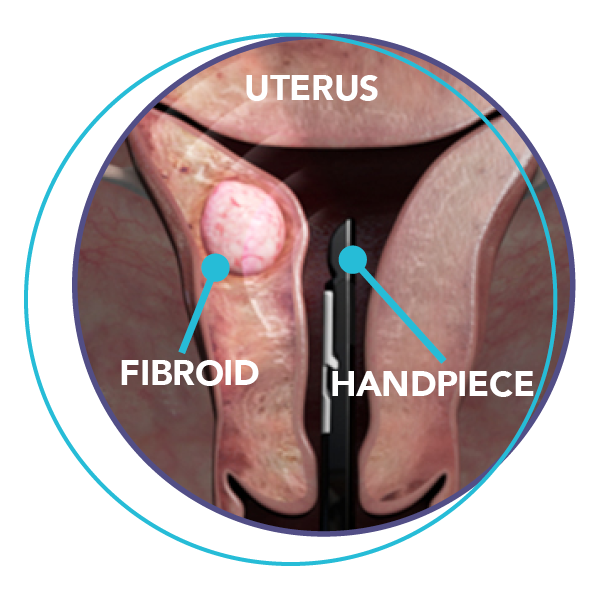
Step 1
The doctor passes the Sonata
Treatment handpiece through
the vagina and into the uterus.
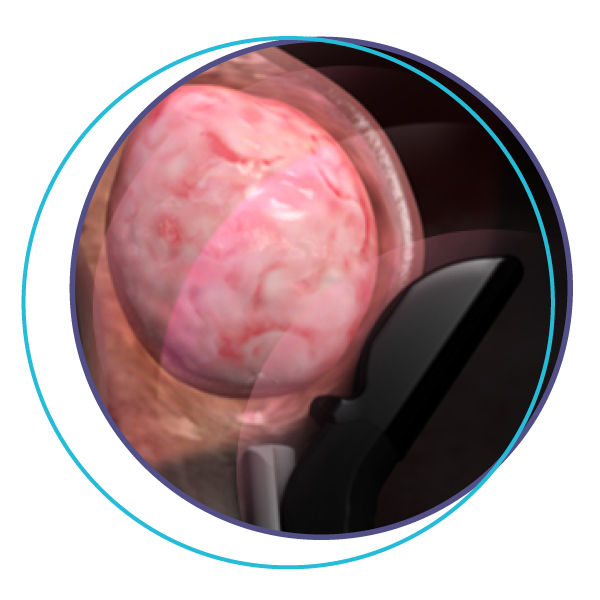
Step 2
Ultrasound waves from the tip
are used to locate the fibroid.
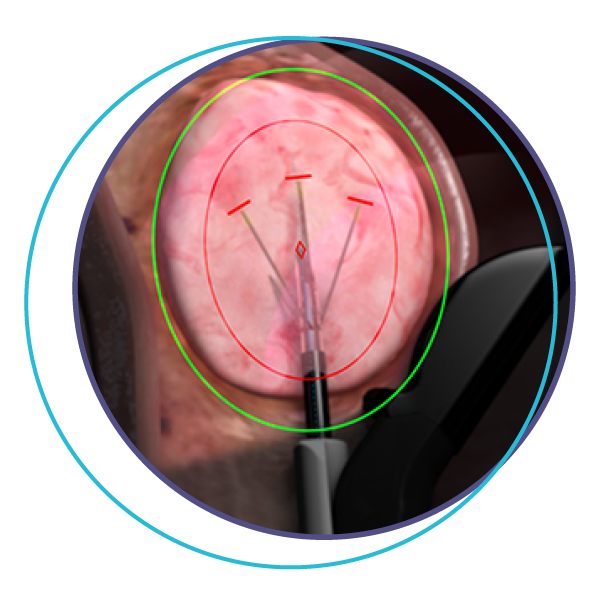
Step 3
The Sonata Treatment handpiece delivers radiofrequency energy to shrink the fibroid over time and reduce symptoms.
Designed to Treat Multiple Fibroid Types in One Incisionless Procedure
Clinical Results
Women were surveyed 12 months after their Sonata Treatment and reported the following results:*
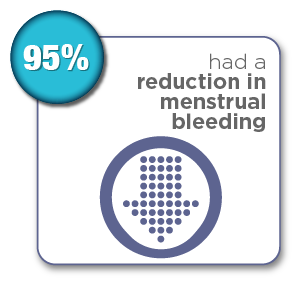
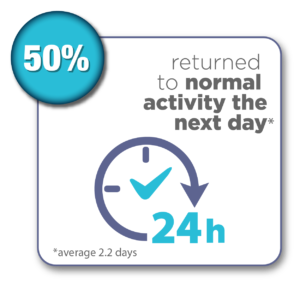
*Chudnoff S, Guido R, Roy K, Levine D, Mihalov L, Garza-Leal JG. Ultrasound-Guided Transcervical Ablation of Uterine Leiomyomas: The SONATA Trial. Obstet Gynecol. 2019 Jan; 133(1): 13-22
Frequently Asked Questions
As of January 1, 2024, Sonata will have a new Category I CPT® code, 58580 (Transcervical ablation of uterine fibroid(s), including intraoperative ultrasound guidance and monitoring, radiofrequency).
The temporary Category III code 0404T (Transcervical uterine fibroid(s) ablation with ultrasound guidance, radiofrequency) will be deleted and no longer accepted as of January 1, 2024.
Give Your Patients the Fibroid Treatment Options They Deserve!
Safety Information | Impressum | Terms of Use | Careers | Contact Us |
Privacy Notice | Cookie Notice | Do not Sell/Share my Personal Information |
Limit the Use of My Sensitive Personal Information
Gynesonics, Inc. | 600 Chesapeake Drive | Redwood City, CA 94063
Copyright 2026 Gynesonics | WS 05195-001 Rev H






